-
Safety of raw ingredients managed in exclusive lines
-
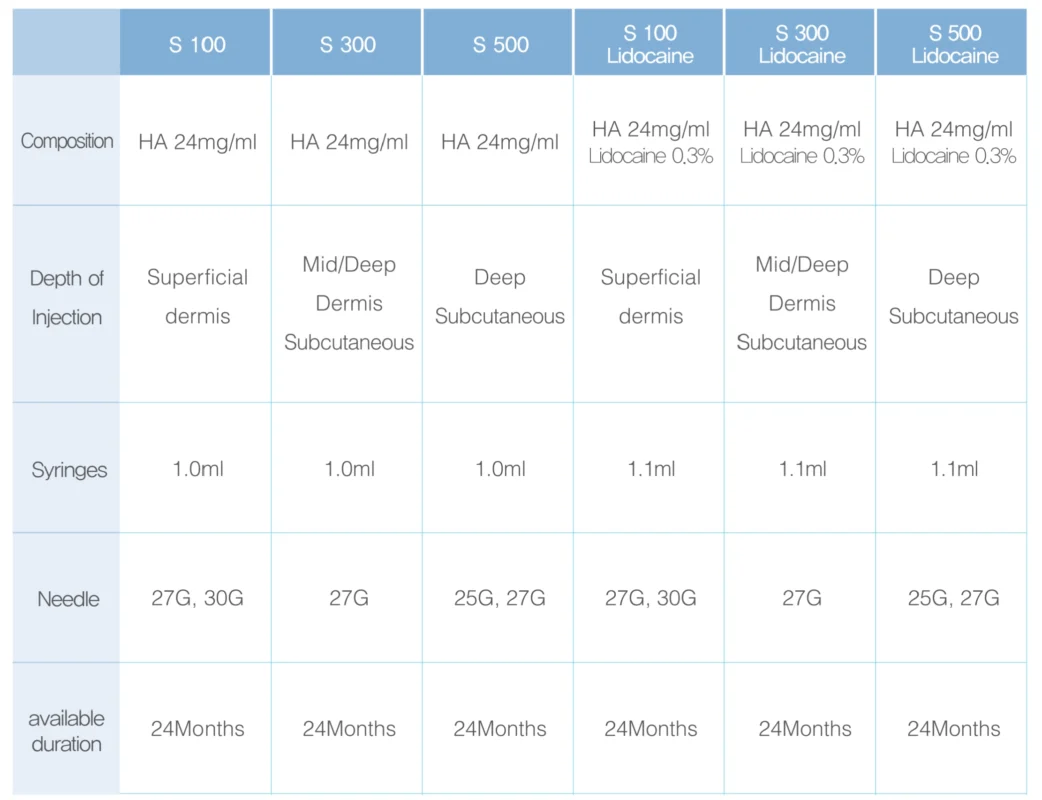
High purity HA content for duration and prevention of swelling
-
High viscoelastic for exquisite lift up
-
Homogeneous particle implementation technology to complete exquisite molding
-
No detection of residual chemical catalyst substance through the complete crosslinking process
-
Aseptic manufacturing environment to prevent side effects
-
Less than 0.1 EU/ml of Endotoxin
-
Minimizes pain with the same pH and osmotic pressure as the human body
-
Optimized injection pressure for exquisite procedures
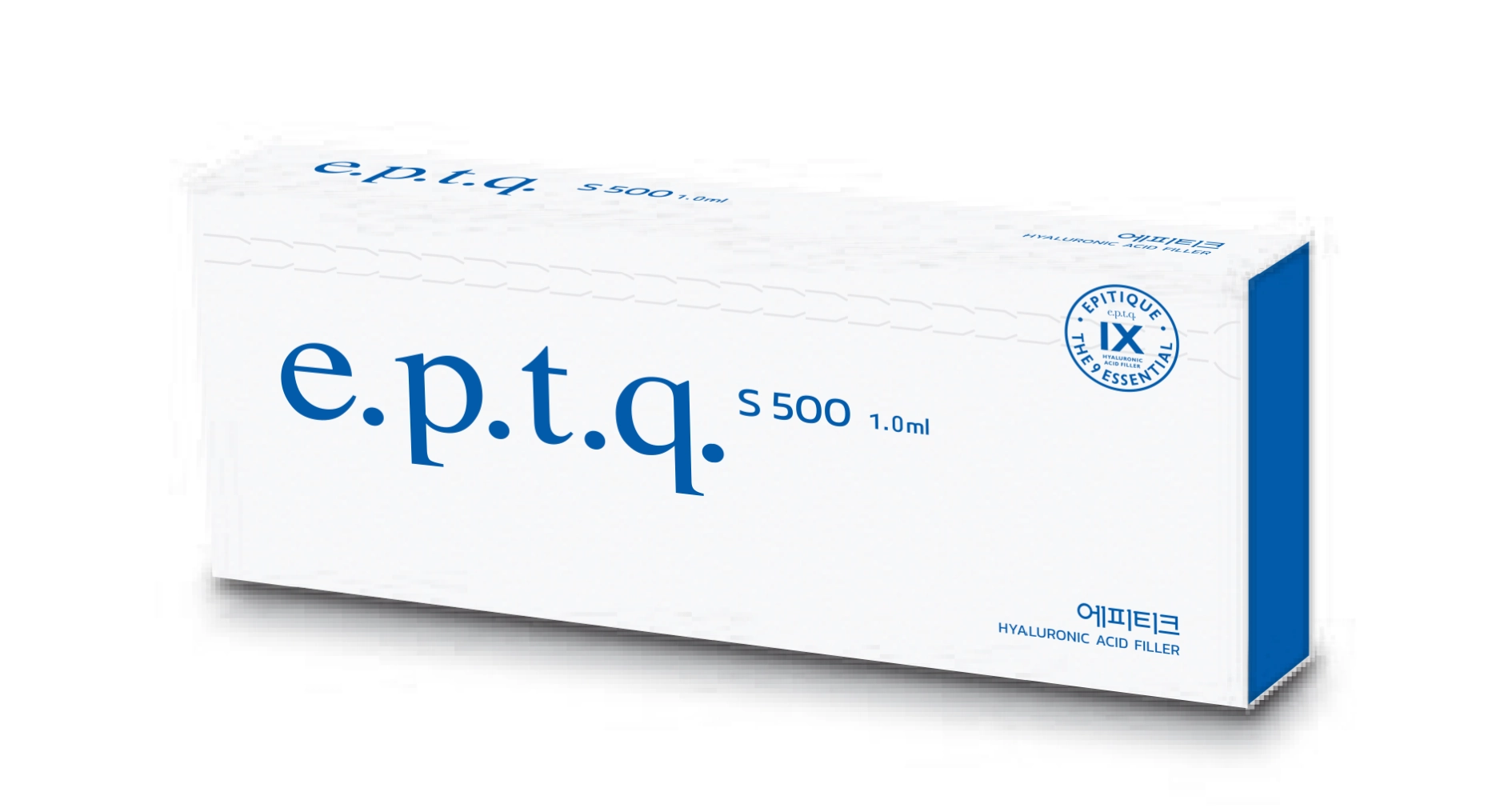
e.p.t.q (epitique)
$38
Product name: Grait/prosthesis, biomaterial (B04230.01 (4))
Product Authorization Number: 17-203
Storage: 2°C~25°C (Do not freeze)
Indication: Temporary improvement of face wrinkles
Single Use / Sterile medical devices / Do not reuse
For more detals, clease refer to the instruction for use.


The IX Essential Process

Brand Story
e.p.t.q. is a word coined from ‘Exquisite’ and ‘Technique.
e.p.t.q. was bom for women who want to manage exquisite detail of beauty to achieve their true self.
Spirit of e.p.t.q.
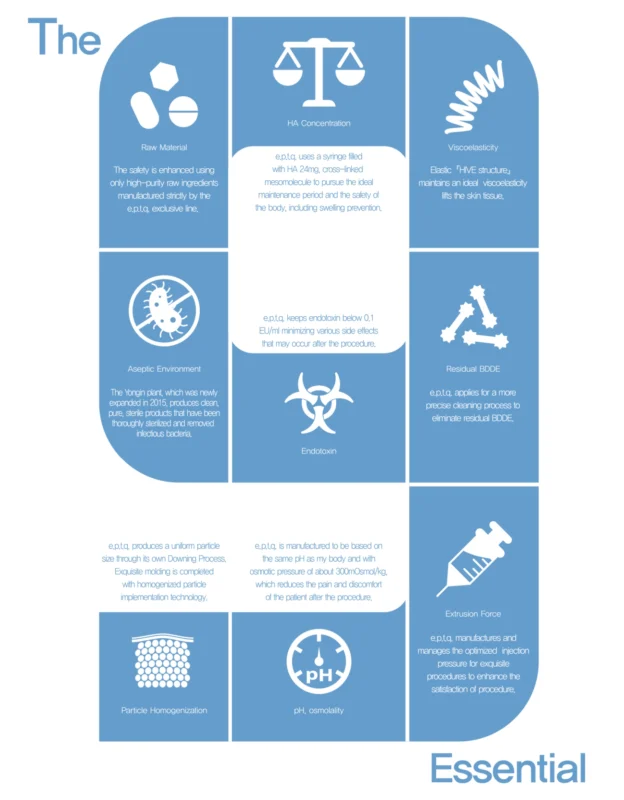
e.p.t.q is manufactured according to “The 9 Essentials” process.
R&D Center has our own 9 criteria to produce high-safety quality HA fillers which is strictly controlled than European standards.
The hyaluronic acid filler e.p.t.q. is produced according to the “The 9 Essentials” process promises an exquisite product that meets stringent safety standards.
9 Reasons Why e.p.t.q. is Exquisite
Exquisite Technology
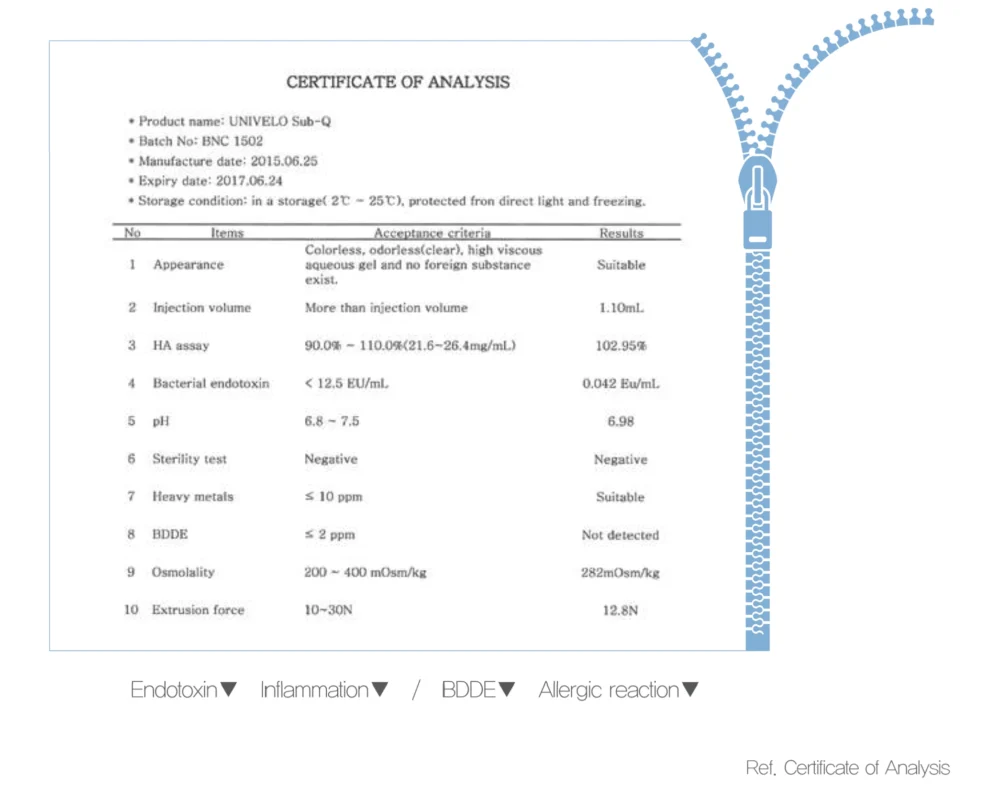
ZEEP (Zero Endotoxin & BDDE Entire Process) Technology
e.p.ta. keeps endotoxin below 0.1 EU/ml and no detection of residual BDDE minimizing various side effects that may occur after the procedure.
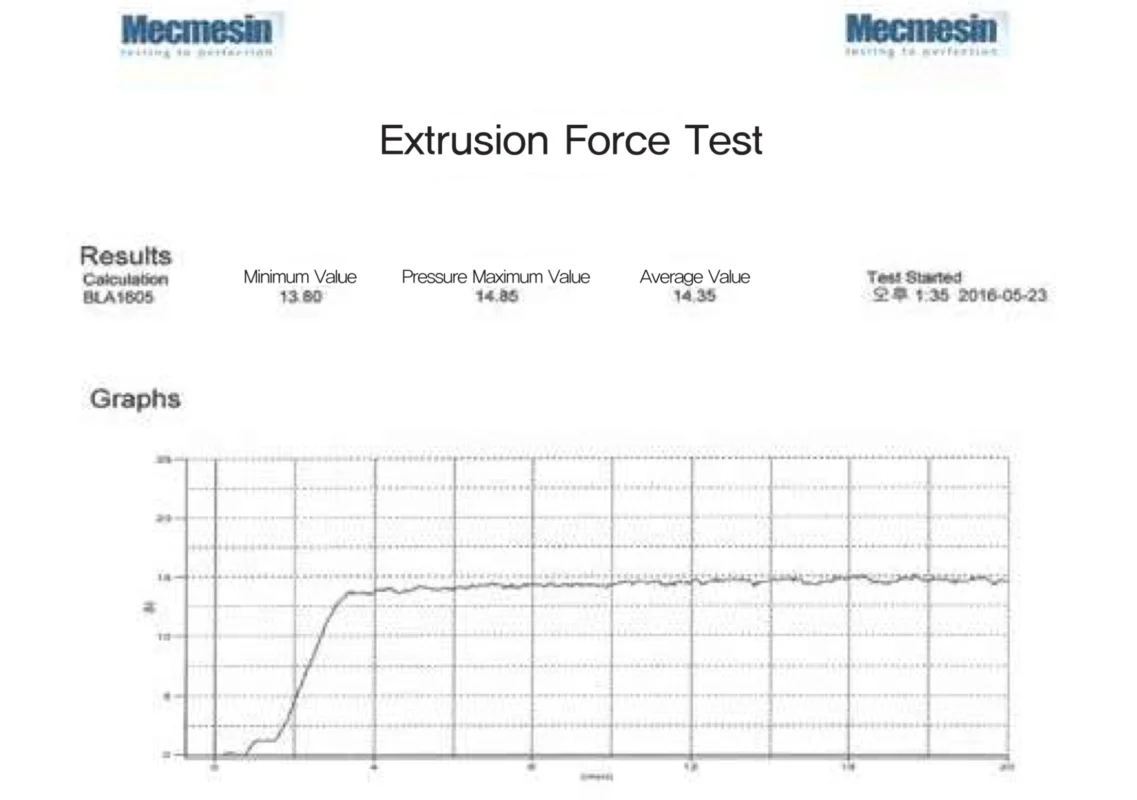
Downing Process
e.p.t.a. produces a uniform particle size through its own Downing Process.
Exquisite procedures are possible without agglomeration through optimized injection pressure.
Exquisite Molding
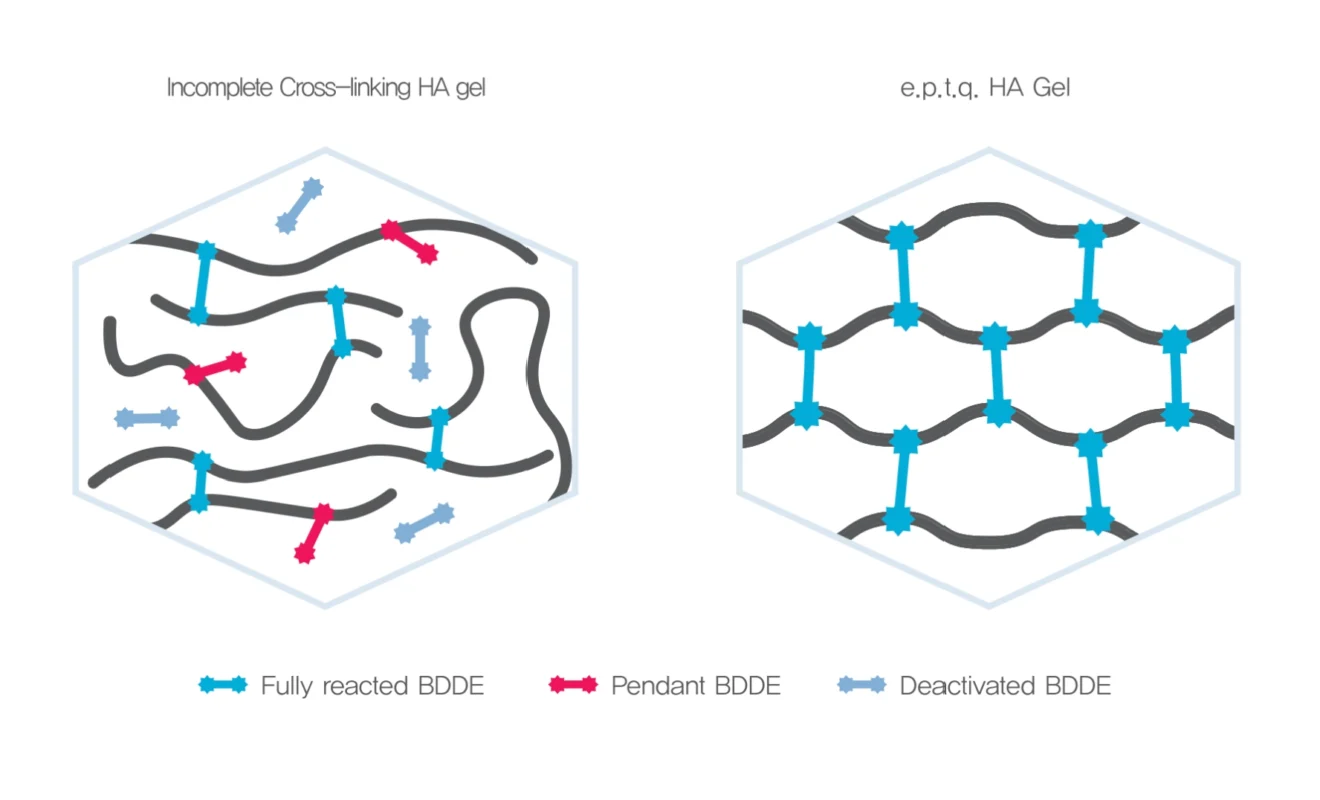
Maximization of Crosslinking Efficiency
e.p.t.q. achieved a full crosslinking level while lowering the BDDE input through an improved crosslinking process.
Its natural result is confirmed after the procedure immediately.
The HIVE Structure of e.p.t.q. HA gel is stable in the skin after injection.
High Viscoelasticity
e.p.t.q. maintains a high complex viscosity between 30~500 Pas with elastic CHIVE structure. .
Ideal viscoelasticity lifts the skin tissue, it is easy to shape, and it keeps naturally at the injection site even with various movements.

Clinical Results
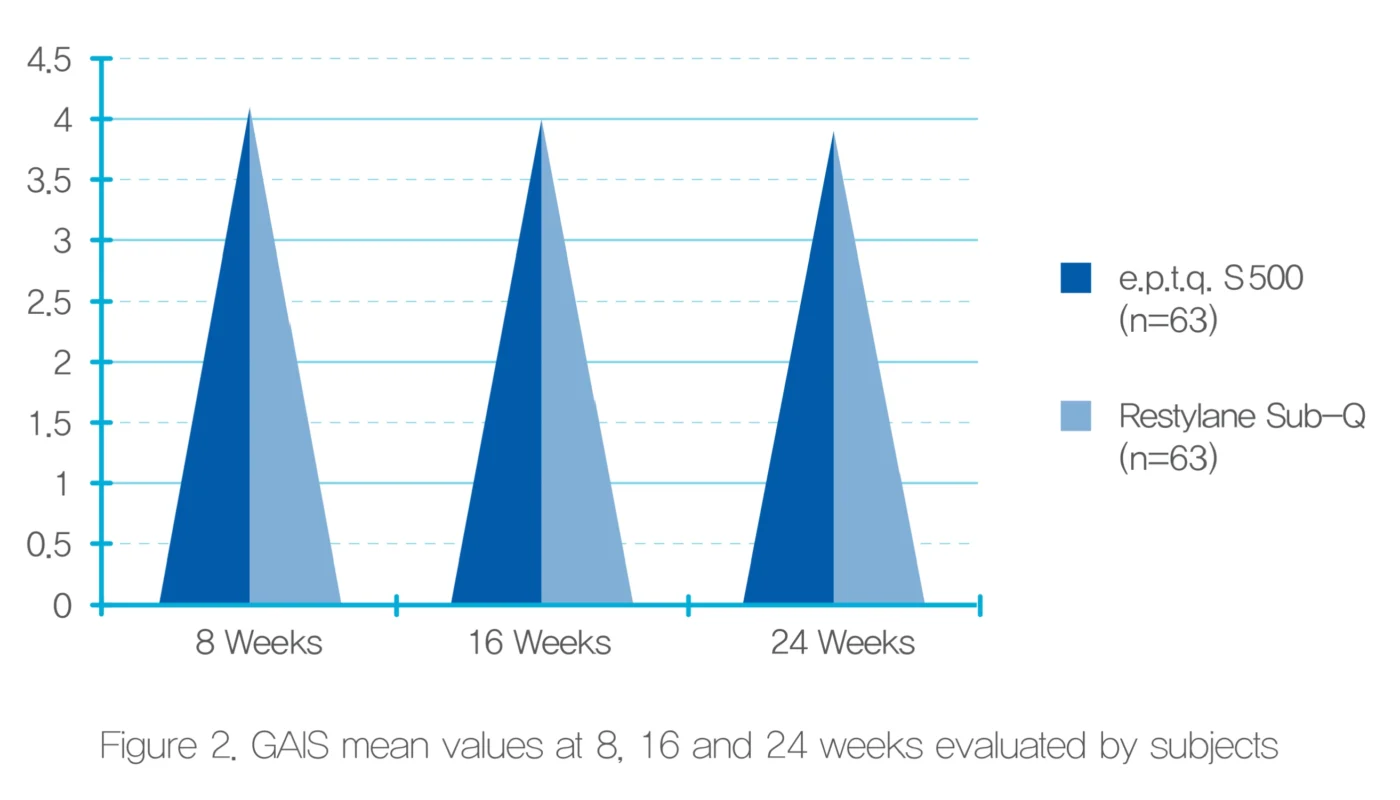
Participant Self – Satisfaction Assessment
Although there was no statistically significant difference in evaluating the satisfaction of the treatment with the tester and the subject’s GAIS, in general, the satisfaction level of the test group was similar or slightly higher than that of the control group.
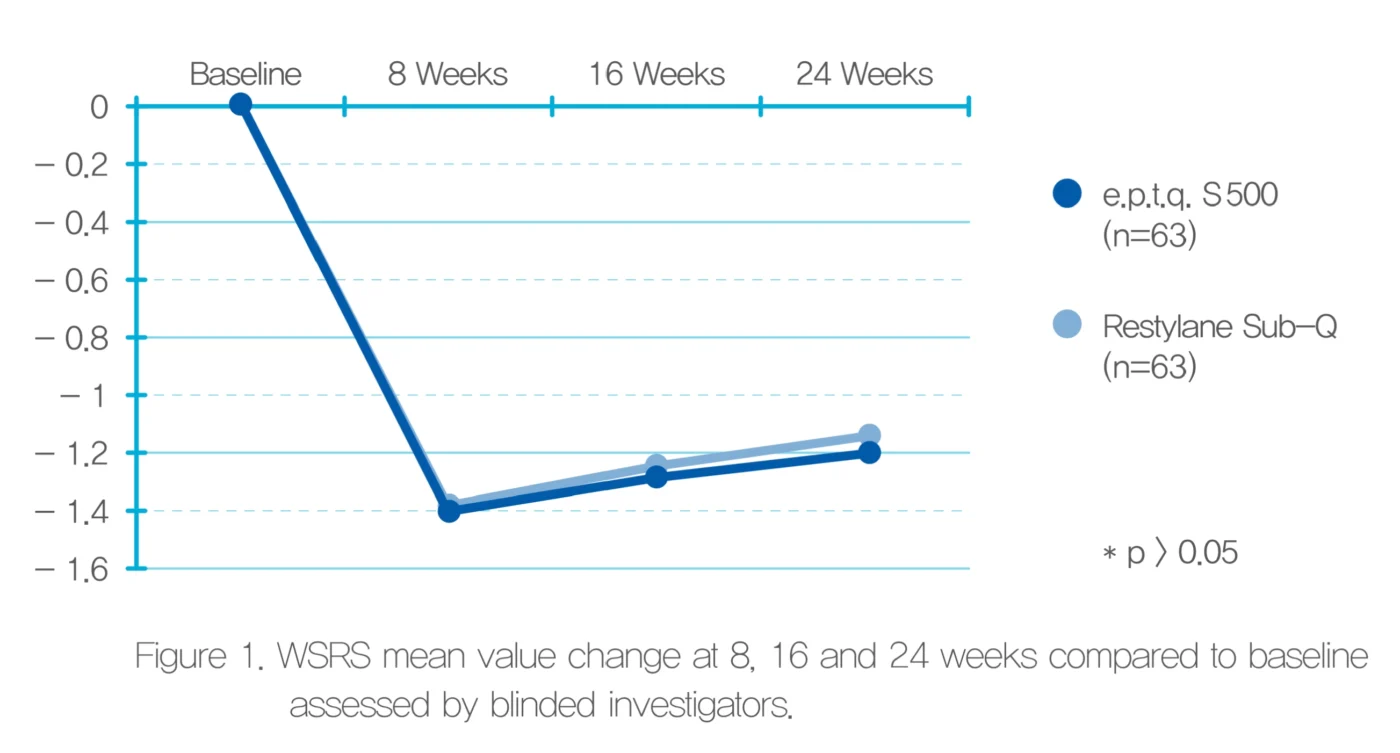
Efficacy Assessment
From the WSRS results (reduced compared to the baseline scores) evaluated by independent expert panels and blinded investigators, we were able to see the wrinkle improvement of the
Safety Assessment
No major abnormalities were reported, and slight abnormal responses from medical devices, such as slight swelling and pain for 2-3 days, were all recovered without any special treatment.
There were no signs of abnormal vital signs, physical examinations, or blood tests. e.p.t.g. has been reported to be a safe medical device due to its proven safety in the body.
Ref. Clinical Study Result, Department of Dermatology. Chung-Ang University Hospital. Korea 2016

Product Information