
WE INCLUDE ICE PACKS THAT MAY MELT DURING SHIPPING. HOWEVER, THE QUALITY OF THE PRODUCTS IS NOT COMPROMISED, AS BRIEF TEMPERATURE CHANGES DO NOT AFFECT THEIR EFFECTIVENESS AND SAFETY

WE INCLUDE ICE PACKS THAT MAY MELT DURING SHIPPING. HOWEVER, THE QUALITY OF THE PRODUCTS IS NOT COMPROMISED, AS BRIEF TEMPERATURE CHANGES DO NOT AFFECT THEIR EFFECTIVENESS AND SAFETY
INNOTOX® is the world’s first liquid formulation of botulinum toxin type A product, an innovative product manufactured without any animal-derived ingredients or human serum albumin.
INNOTOX® is easy to use since no dilution is necessary, which minimizes potential hygiene problems in preparation process.
Efficacious and safety of INNOTOX® are proven in clinical study for improvement of glabella wrinkles.
It is easy to measure accurate dose for treatments (INNOTOX® 0.1mL = 4unit)
.
As the first company successfully developed botulinum toxin in Korea, Medytox’s R&D capability sets the new standard of global botulinum toxin market.
.
INNOTOX® – the product of world-class R&D capability
As the first company successfully developed botulinum toxin in Korea, Medytox’s R&D capability sets the new standard of global botulinum toxin market.

INNOTOX®
Clostridium botulinum toxin type A, L-methionine, polysorbate 20, Sodium chloride (9mg)
It appears as a colorless transparent solution for injection in a colorless transparent vial.
Store in a refrigerator (2~8°C).
INNOTOX® is supplied in a single-use vial.
10U (2.5 ml/vial) : 18 months from the manufacturing date.
INNOTOX® is for the temporary improvement of moderate to serious glabella wrinkles related to corrugator muscle and/or procerus muscle activity in adults over the age of 20 and under the age of 65.
* One unit(U) of INNOTOX® corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice..
Next Generation of Botulinum Toxin Type A INNOTOX®
INNOTOX® is the first liquid formulation of botulinum toxin type A product in the world, produced without any animal-derived
![]() INNOTOX® manufacturing process
INNOTOX® manufacturing process
Clostridium
Botulinum
Toxin
Type A
Culture in media without animal-derived ingredients
Use non-animal based stabilizer (Excluded HSA)
Filling
INNOTOX®
(Liquid type)
![]() Technologies used in INNOTOX® manufacturing are highly commented globally, and secured the biggest license-out contract in pharmaceutical industry of Korea2)
Technologies used in INNOTOX® manufacturing are highly commented globally, and secured the biggest license-out contract in pharmaceutical industry of Korea2)
Reference:
1) Susan A. Wang et al. Enterobacter cloacae Bloodstream Infections Traced to Contaminated Human Albumin. 2000 by the Infectious Diseases Society of America.
2) Public announcement at Sep. 2013(http://www.nasdaq.com/article/allergan-inks-deal-with-medytox-analyst-blog-cm280570)
The manufacturing of INNOTOX® completely excludes animal-derived ingredients and human serum albumin, which enhances long-term safety.1)
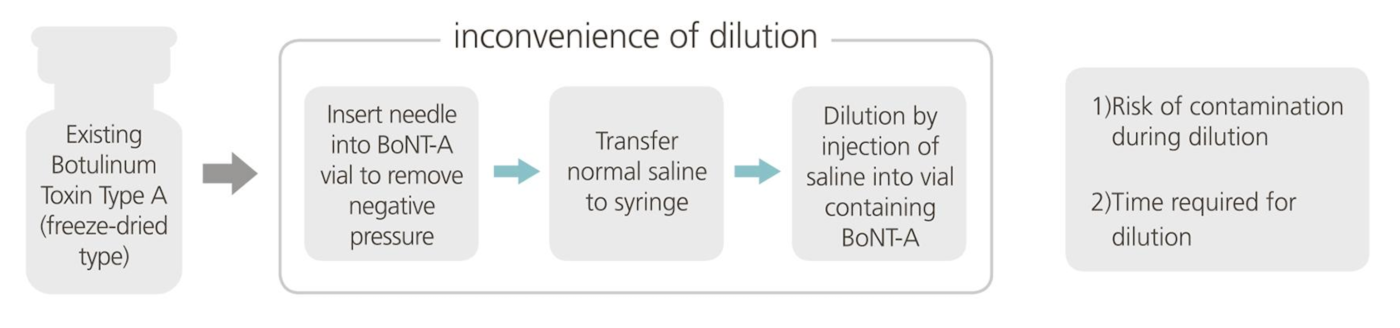
User Friendly, INNOTOX®
INNOTOX® is easy to use since no dilution is necessary, which minimizes potential hygiene problems.
![]() Preparation of procedure
Preparation of procedure

Without dilution procedure,
INNOTOX® can be used directly for injection
Proven Efficacy & Safety INNOTOX®
Efficacy and safety of INNOTOX® are proven in clinical study for improvement of glabella wrinkles.
When same administration method and dose were used, effect of both products on improvement of glabella wrinkles evaluated by investigator at 4 weeks after treatment was the same.
However, the effect of INNOTOX® was significantly different at 16 weeks after treatment.
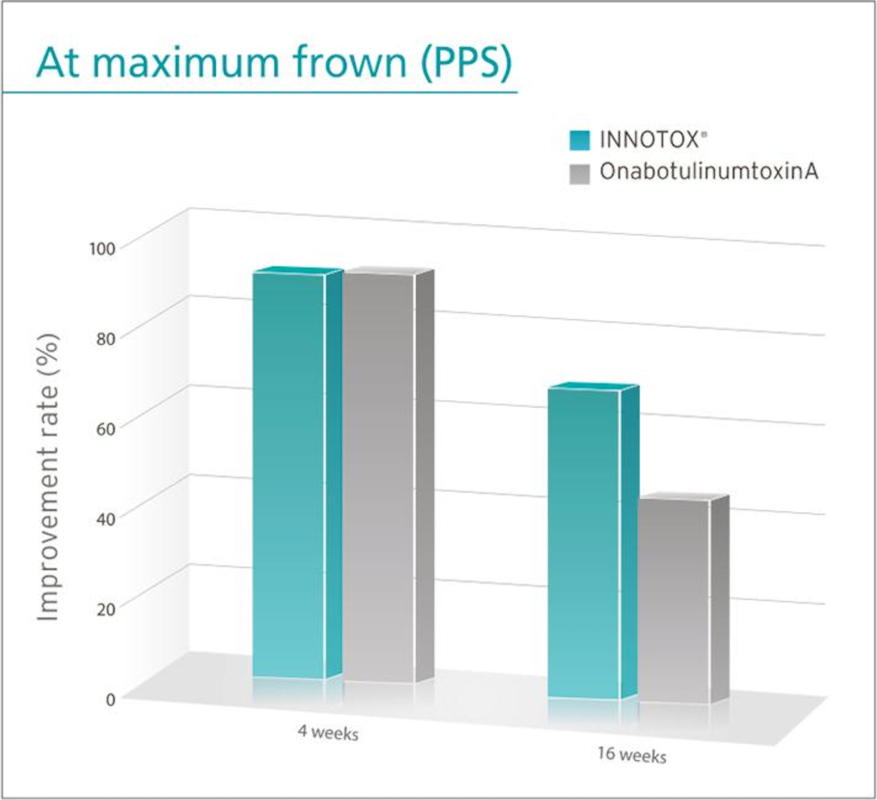
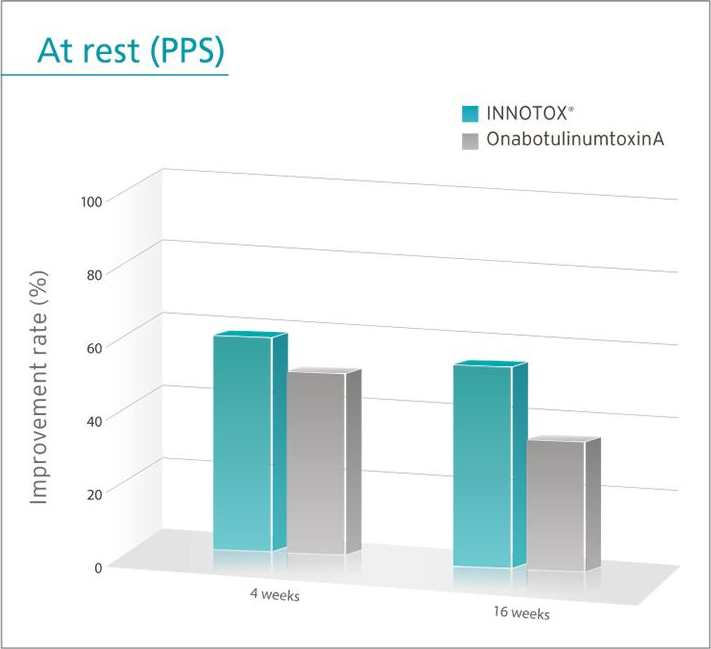
• PPS: Per Protocol Set
* The result of this clinical study was adopted by American Society for Dermatologic Surgery and presented in October 2013.
Reference: Plast. Reconstr. Surg. Journal. 135: 732-741, 2015.
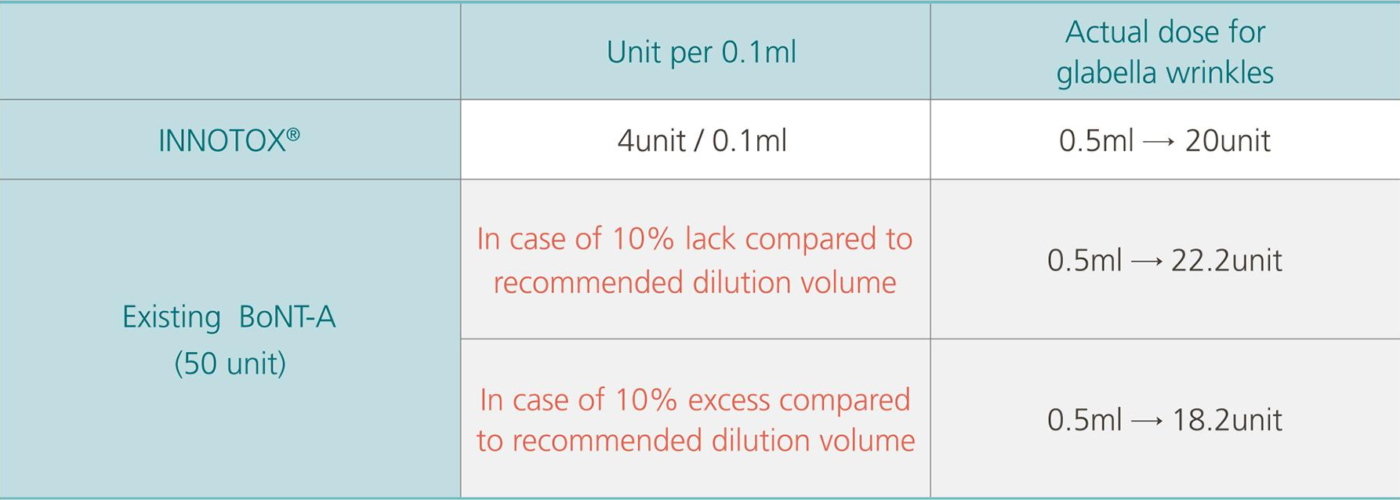
Accurate Dose, INNOTOX®
It is easy to measure accurate dose for treatment.
![]() Eliminate possibility of inaccurate dilution volume by user’s handwork (INNOTOX® 0. 1mL = 4unit)
Eliminate possibility of inaccurate dilution volume by user’s handwork (INNOTOX® 0. 1mL = 4unit)
$
