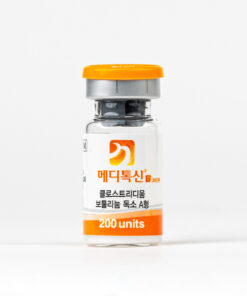
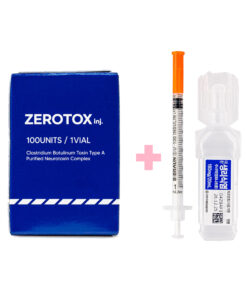
IMPORTANT
THE VIAL MAY SEEM EMPTY
−
AND THAT’S NORMAL!


WE INCLUDE ICE PACKS THAT MAY MELT DURING SHIPPING. HOWEVER, THE QUALITY OF THE PRODUCTS IS NOT COMPROMISED, AS BRIEF TEMPERATURE CHANGES DO NOT AFFECT THEIR EFFECTIVENESS AND SAFETY