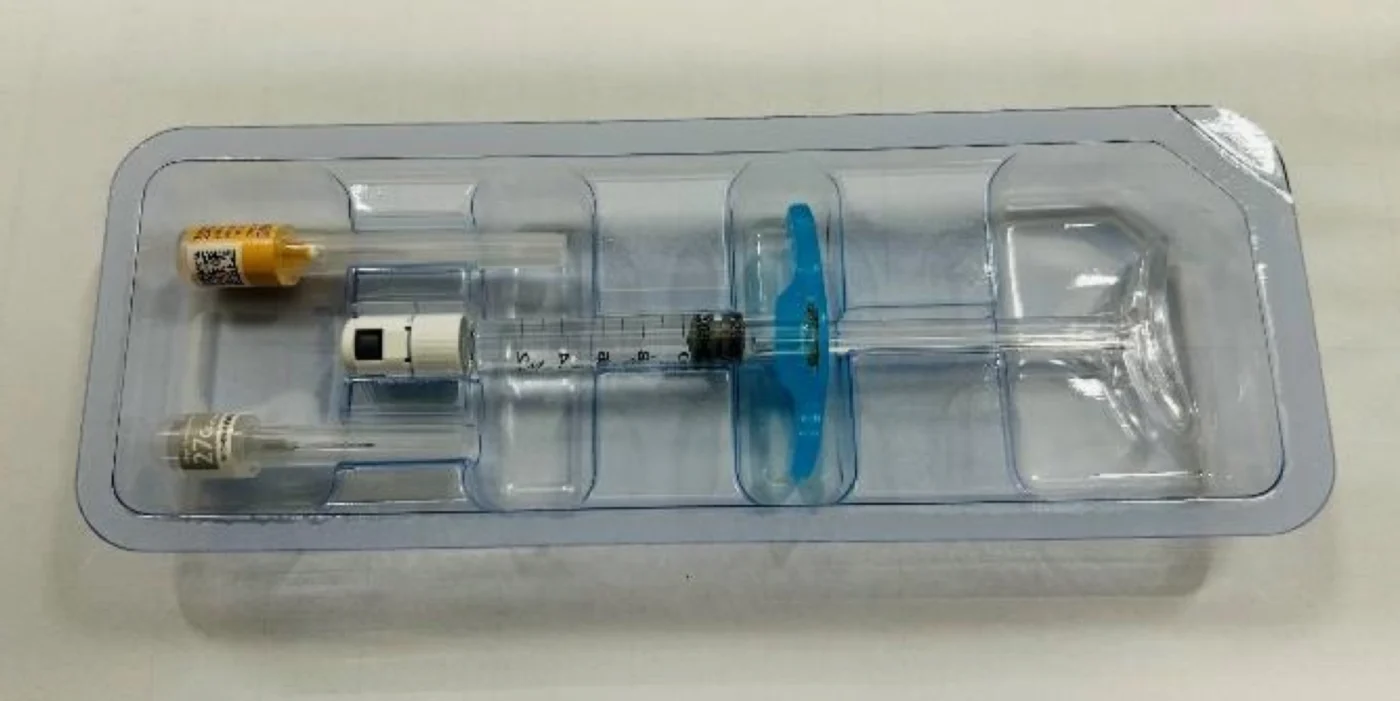
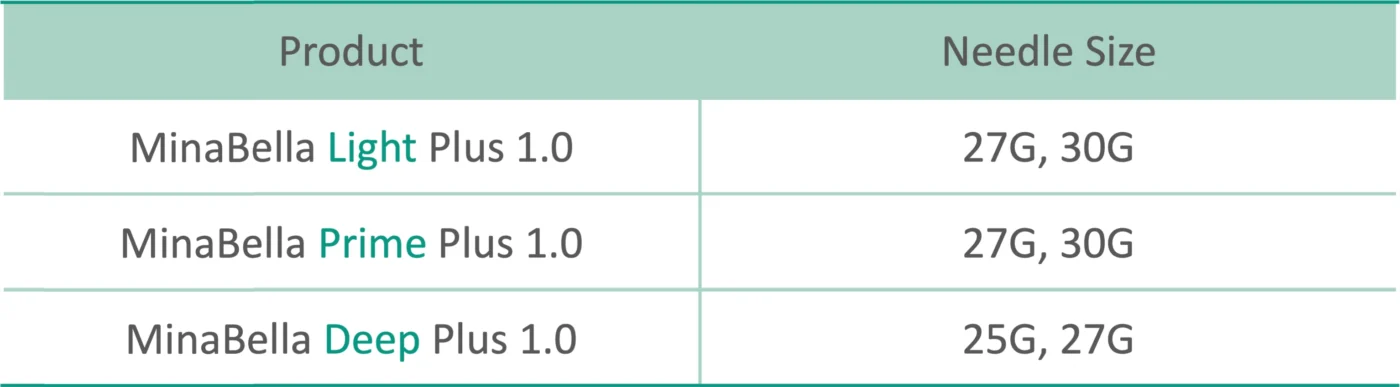
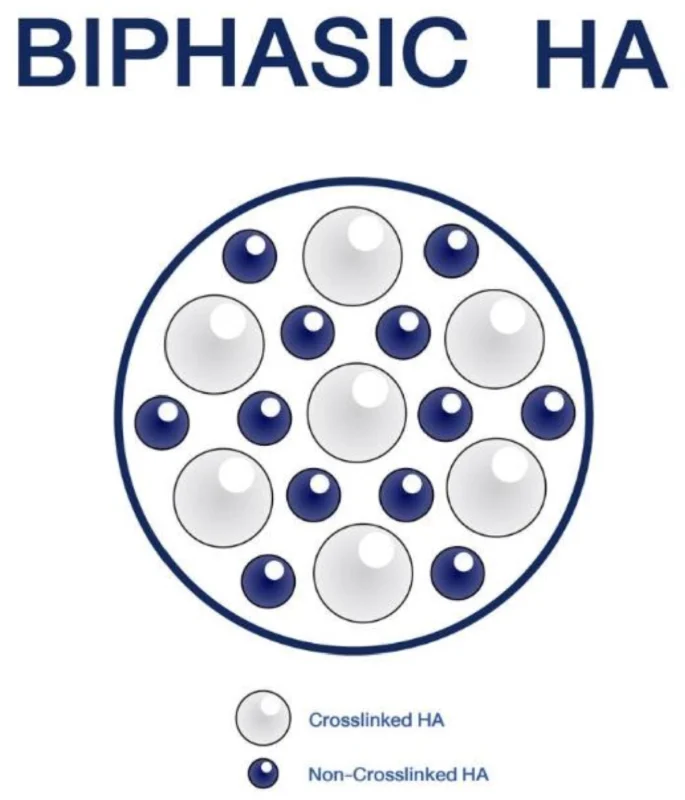
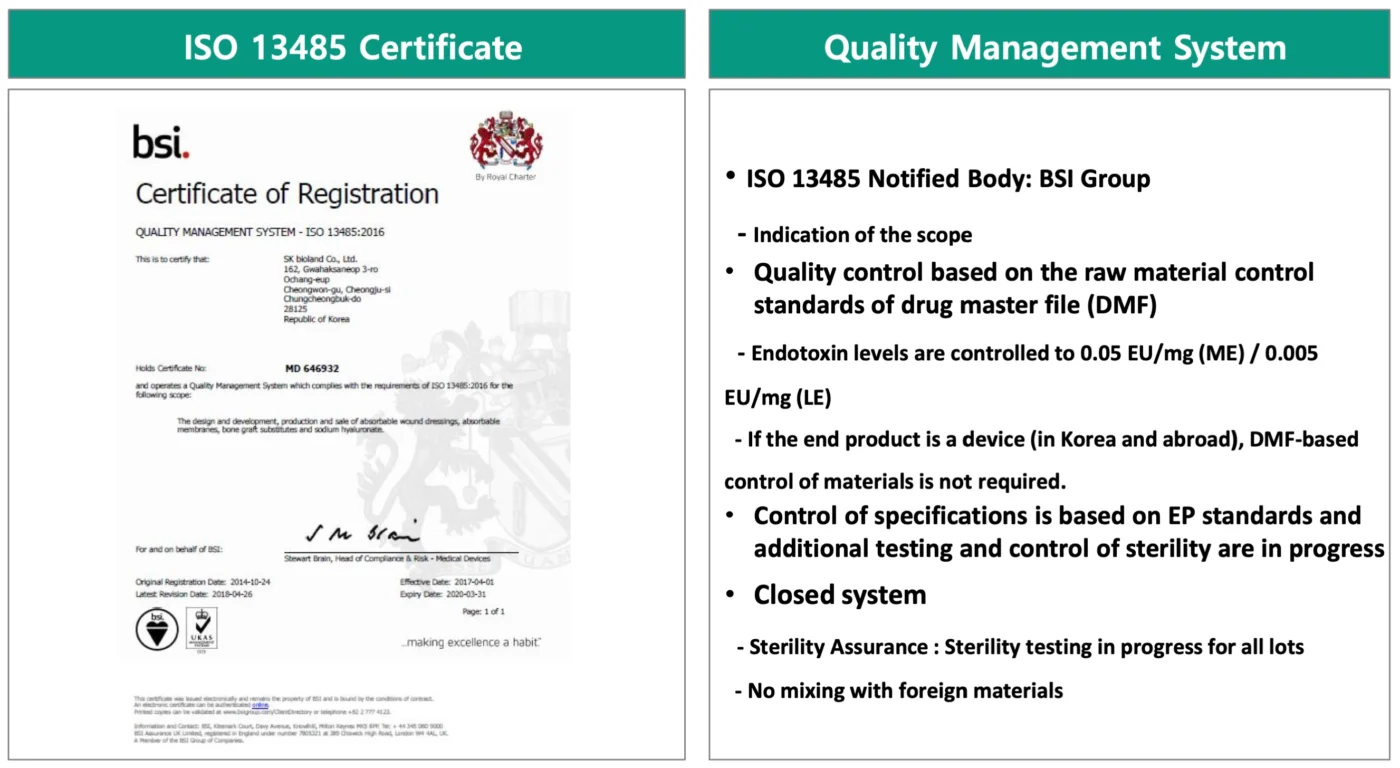
Introducing MinaBella
MinaBella has 3 different product lines so it can be strategically injected in the right areas and gives volume to the skin.
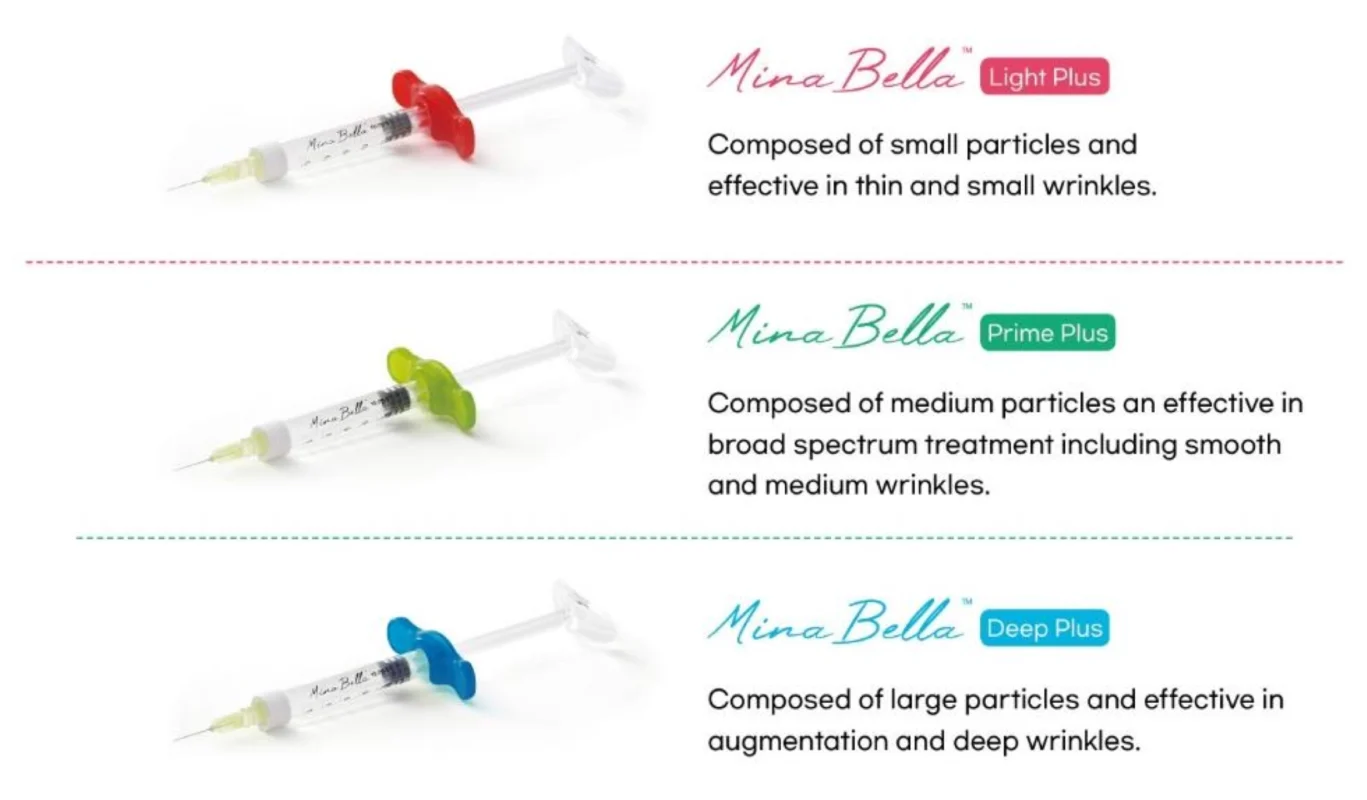
* HA derivatives with concentrations adjusted are ground in plunger mills to produce different particle sizes.