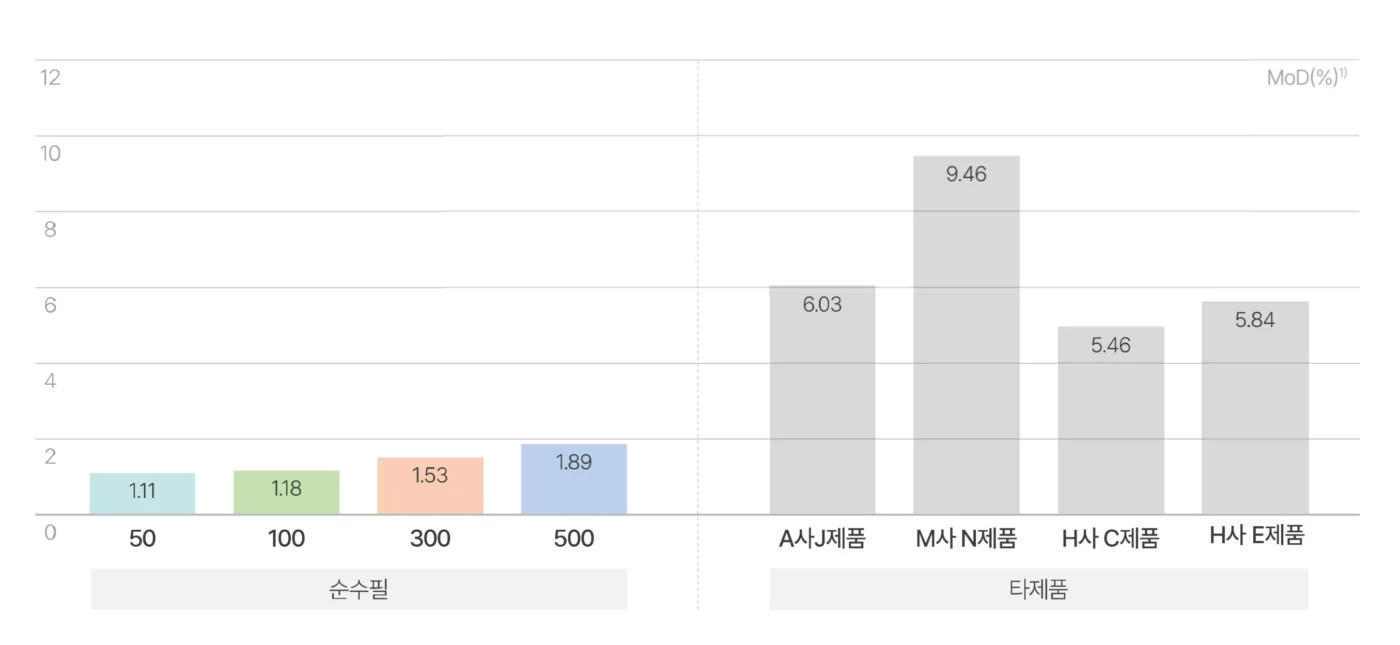
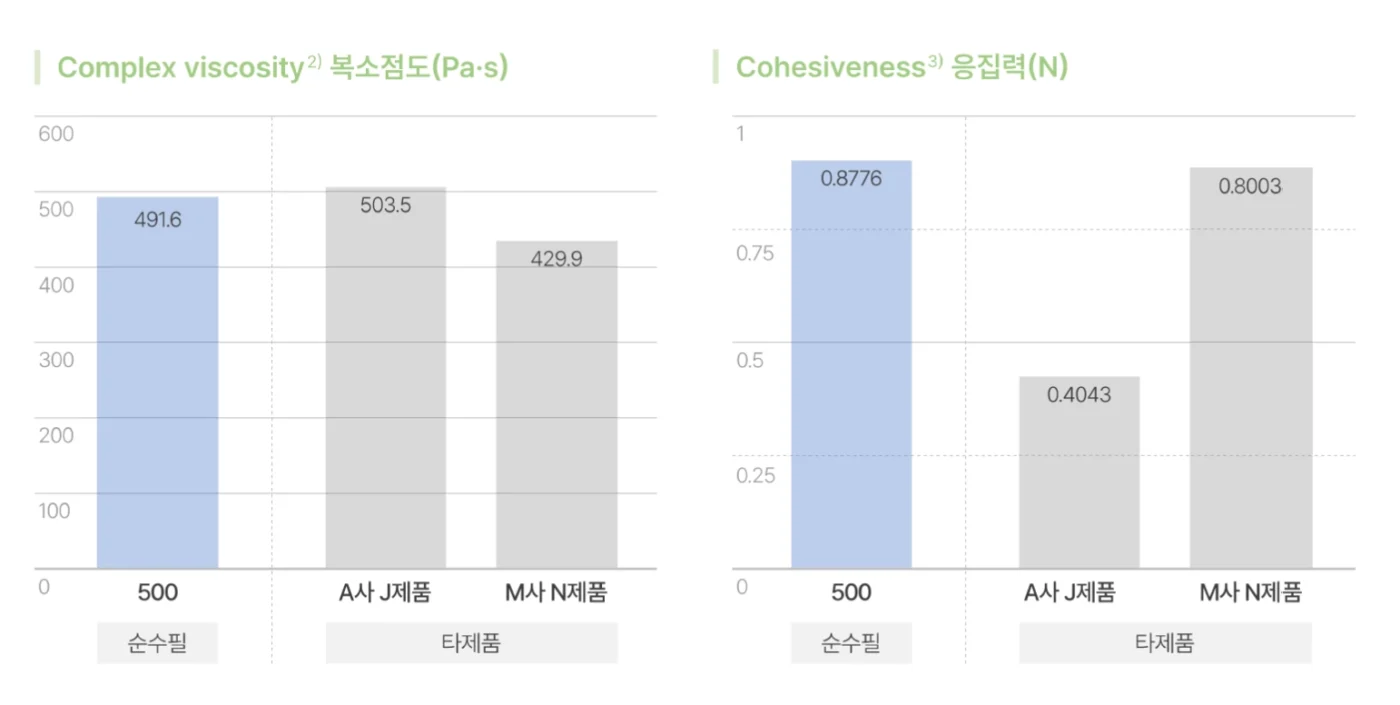
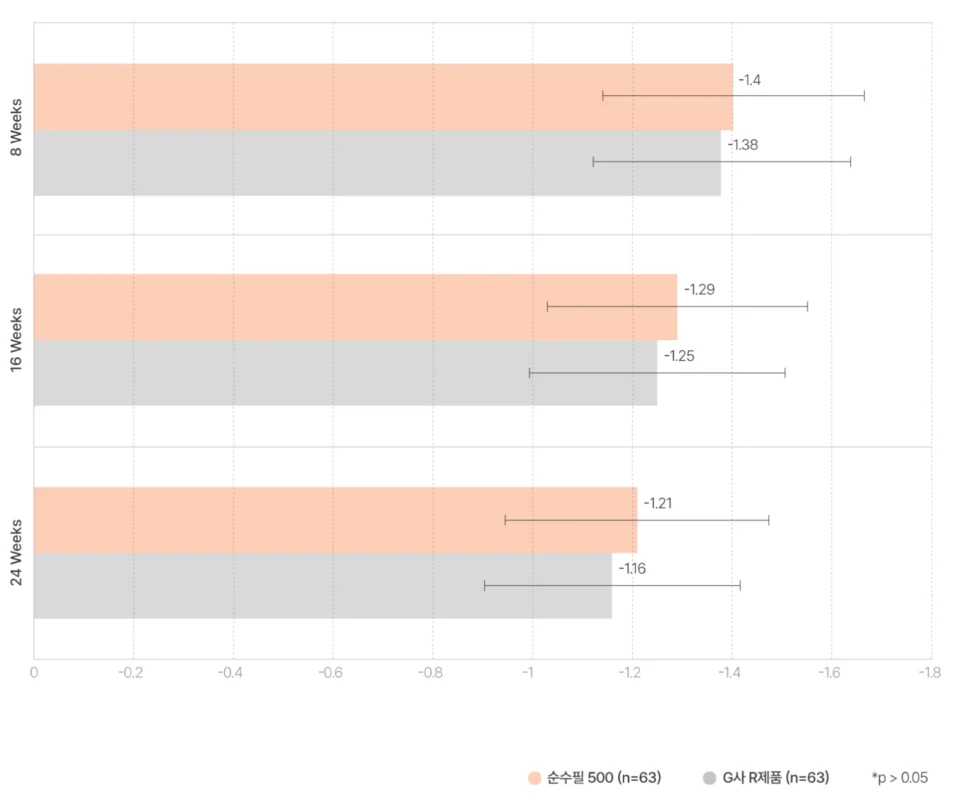
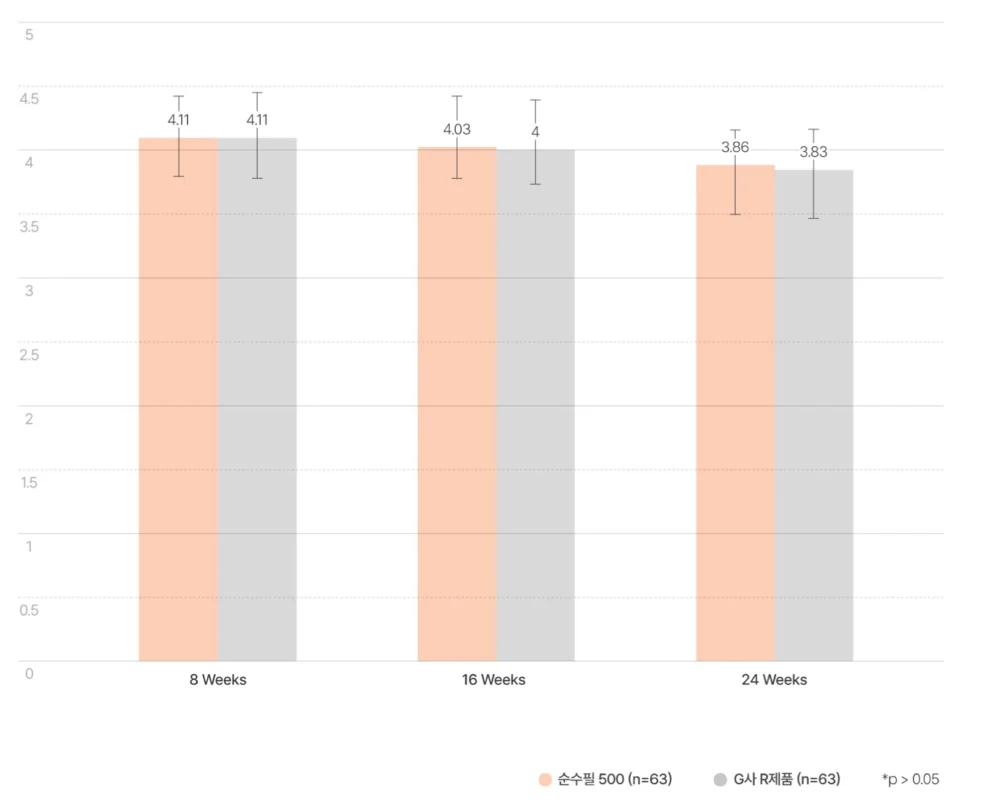
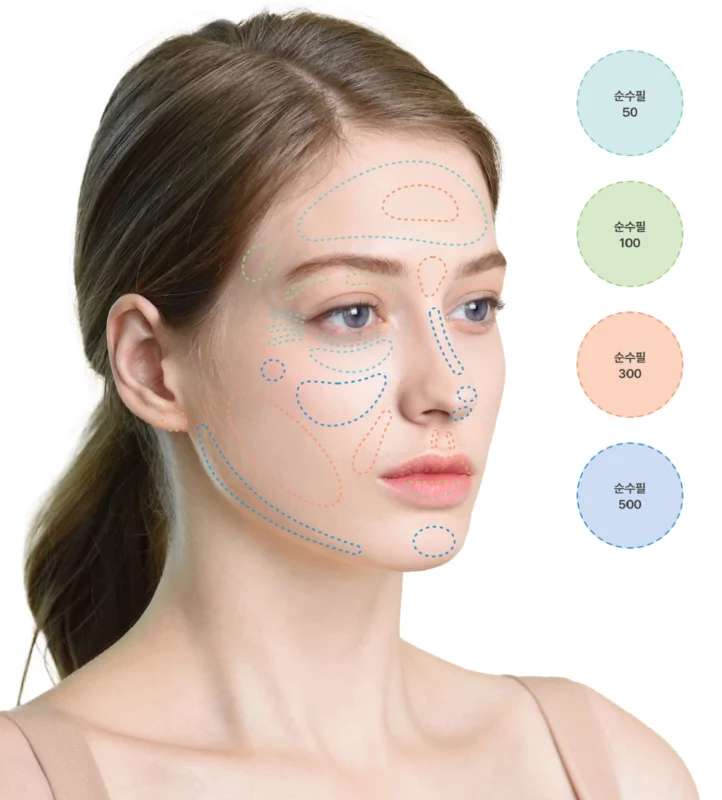
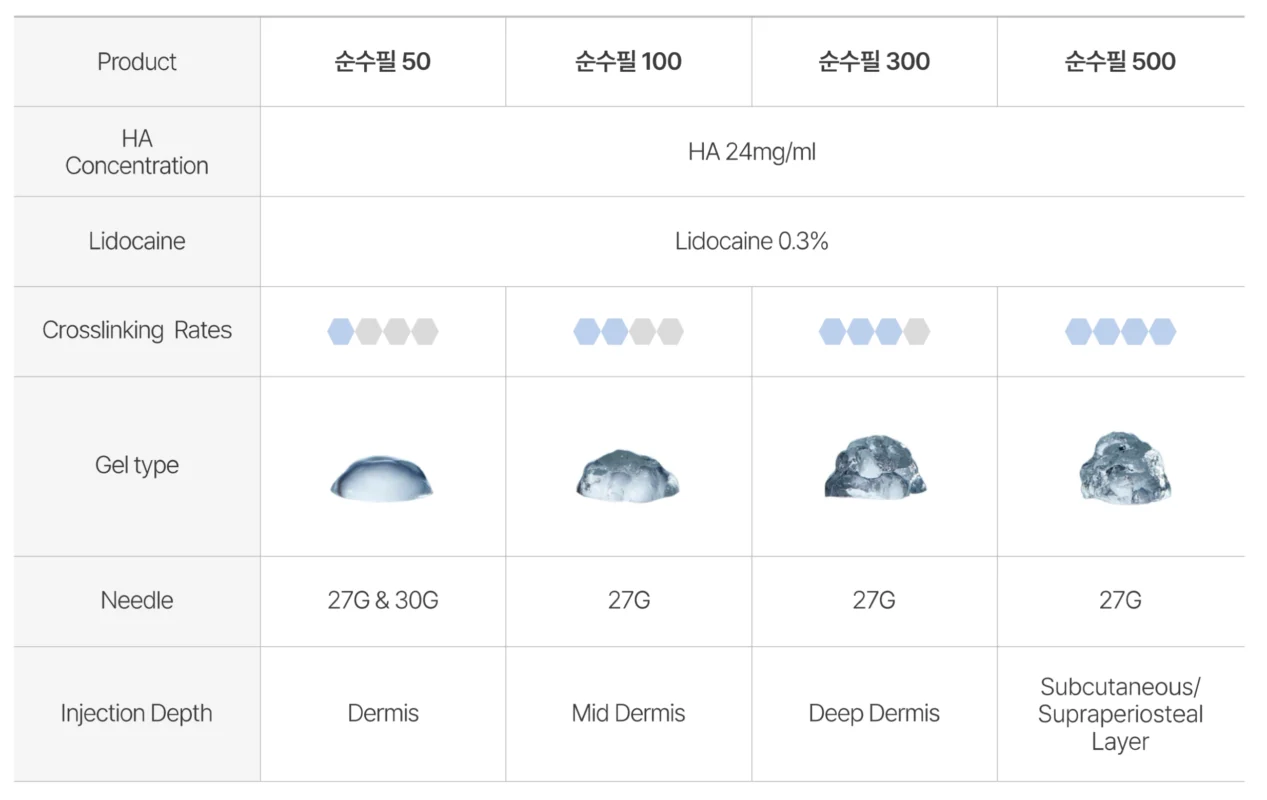
순수필
HYALURONIC ACID FILLER WITH LIDOCAINE
Soosoofill is reborn with a name that not only carries on the proven safety of Epitique® fillers, but also strengthens the unique identity of our brand.
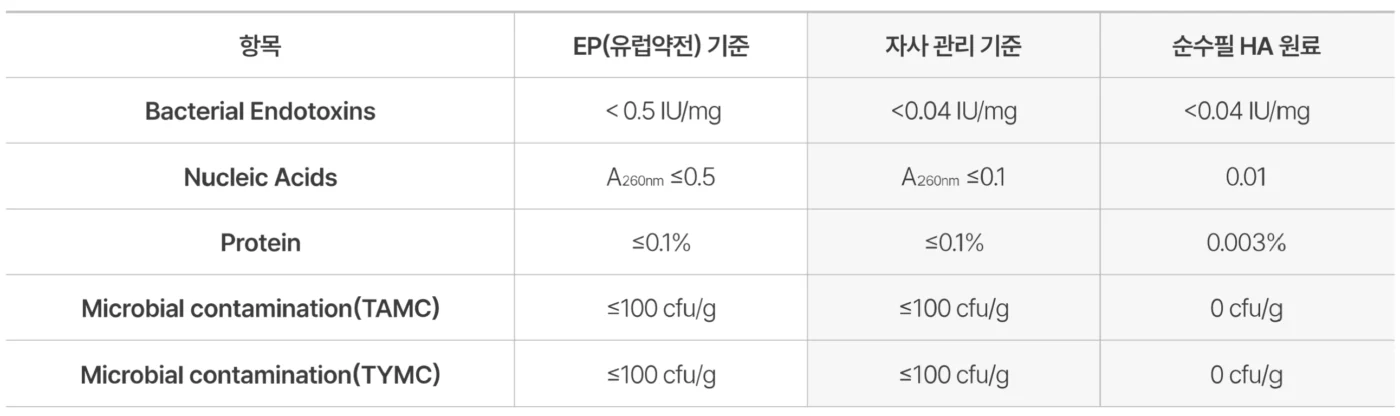
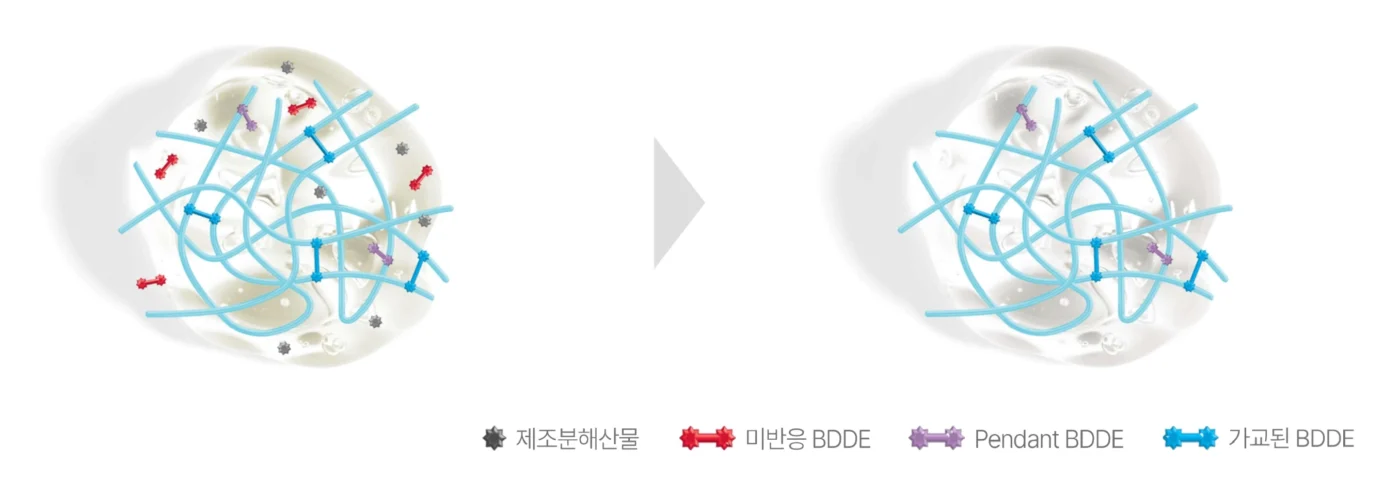
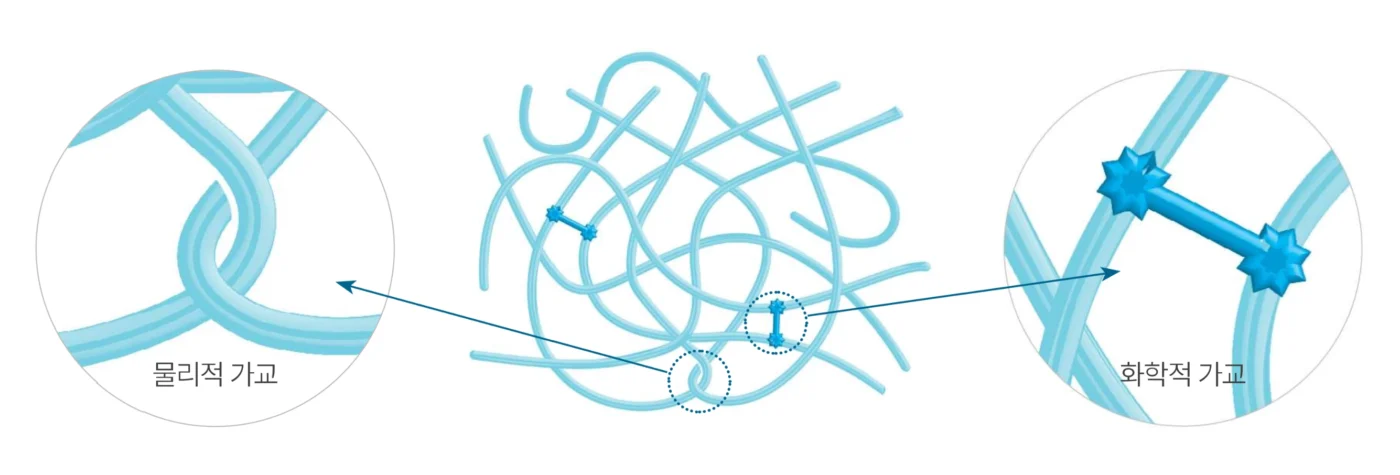
Crafted through rigorous sterilization and advanced purification, Soosoofill hyaluronic acid filler promises exceptional safety and purity — made only with the highest-quality ingredients you can trust.